ANATOMICAL HEART
Right lateral thoracic radiograph of a small dog in ACVIM Stage C, heart failure.
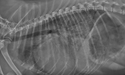
Image courtesy of Rebecca L. Stepien, DVM, MS, DACVIM (Cardiology), University of Wisconsin, USA
Heart Failure
Heart disease invariably progresses, but it does not always lead to heart failure. The prognosis depends on the pet’s overall health, the type of heart disease and its rate of progression.1
Heart failure refers to the clinical signs – such as fluid accumulation in the lungs or abdomen – that occur when the heart can no longer adequately compensate for changes associated with the heart disease.
When heart failure develops, current nutritional recommendations focus on correcting nutrient deficiencies, managing clinical signs, and maintaining adequate caloric and protein intake to preserve lean body mass and avoid cachexia.
At the cellular level, heart failure could be called an energy crisis. The mitochondria become dysfunctional, energy metabolism is inefficient, and cardiac contractility diminishes.2
Learn more about the bioenergetic changes in heart failure that provide opportunities for new nutritional approaches to cardiac health.
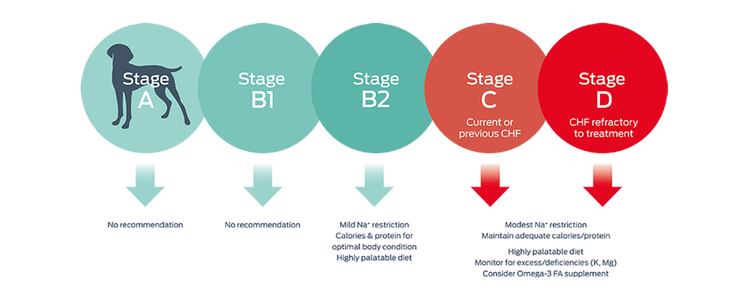
The clinical signs of heart failure are hard to miss: lethargy, tachypnea, a cough, or a fluid-filled abdomen. At this late stage of heart disease, ACVIM guidelines make several nutritional recommendations.1
But changes at the cellular level are not so easy to see.
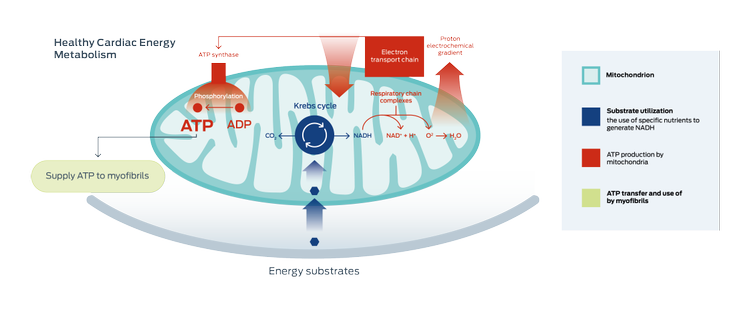
Compromised cardiac energy metabolism is a key aspect of heart failure.2-5
In general, energy metabolism in cardiac mitochondria has three components:2-5
- The use of energy substrates (fatty acids, glucose, and other nutrients)
- Energy (ATP) production
- ATP transport to, and use by, the myofibrils (heart muscle)
In heart failure, studies show that changes can occur in any, or all three, areas of energy metabolism.2
When cardiac function declines, any one area of altered energy metabolism leads to negative impacts on every other aspect of ATP production.
Ultimately, the failing heart has an energy crisis.2, 6, 7
Studies show that mitochondria in the failing heart change the substrates used to produce energy. The overall process of mitochondrial energy production becomes less efficient.
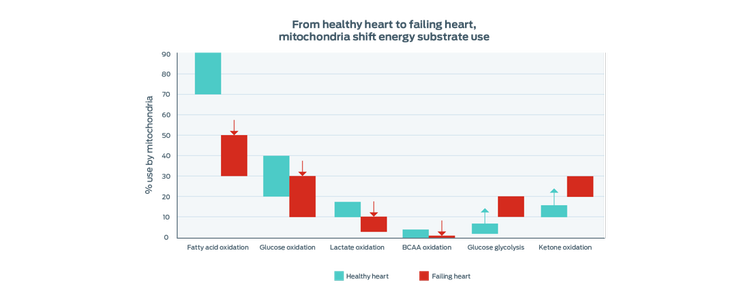
Adapted from Lopaschuk 2017
Purina's research

Omics studies, in both people and animals, have shown that gene expression and metabolite profiles associated with energy metabolism differ significantly between healthy hearts and those with heart failure.8–10
Purina scientists found more than 1,000 gene transcripts were differentially expressed between healthy dogs and those with MMVD. The genes expressed in MMVD dogs were associated with altered pathways in:
- Energy metabolism
- Oxidative stress
- Inflammatory mediators
- The mitral valve’s extracellular matrix homeostasis
Similar to findings in studies of human heart failure, these changes suggest that MMVD dogs also make a metabolic shift away from using long-chain fatty acids as a primary energy source and there is less efficient use of energy overall.
These omics study insights, and emerging research in therapeutic nutrition,12 suggest that providing alternate sources of energy may help with management of heart disease.
Key things to remember
- Heart disease does not always lead to heart failure. The prognosis depends on the disease, its rate of progression, and the pet’s overall health.
- Heart failure refers to the clinical signs that occur when the heart can no longer compensate for changes associated with the heart disease.
- Studies show that cardiac energy metabolism differs significantly between healthy hearts and those in heart failure.
- Research suggests that nutrients providing alternate energy sources for cardiac mitochondria could transform the management of cardiac health.
Explore areas of transforming heart health:
Find out more
- Keene, B. W., Atkins, C. E., Bonagura, J. D., Fox, P. R., Häggström, J., Fuentes, V. L., Oyama, M. A., Rush, J. E., Stepien, R., & Uechi, M. (2019). ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. Journal of Veterinary Internal Medicine, 33(3), 1127–1140.
- Neubauer, S. (2007). The failing heart – an engine out of fuel. The New England Journal of Medicine, 356(11), 1140–1151.
- Lopaschuk, G. (2017). Metabolic Modulators in Heart Disease: Past, Present, and Future. Canadian Journal of Cardiology, 33, 838–849.
- Sabbah, H. N. (2020). Targeting the Mitochondria in Heart Failure: A Translational Perspective. JACC. Basic to Translational Science, 5(1), 88–106.
- Taegtmeyer, H. (2004). Cardiac metabolism as a target for the treatment of heart failure. Circulation,110(8), 894–896.
- Doenst, T., Nguyen, T. D., & Abel, E. D. (2013). Cardiac metabolism in heart failure: implications beyond ATP production. Circulation Research, 113(6), 709–724.
- Karwi, Q. G., Uddin, G. M., Ho, K. L., & Lopaschuk, G. D. (2018). Loss of Metabolic Flexibility in the Failing Heart. Frontiers in Cardiovascular Medicine, 5, 68.
- Li, Q., Freeman, L. M., Rush, J. E., Huggins, G. S., Kennedy, A.D., Labuda, J.A., Laflamme, D.P., & Hannah, S.S. (2015). Veterinary Medicine and Multi-Omics Research for Future Nutrition Targets: Metabolomics and Transcriptomics of the Common Degenerative Mitral Valve Disease in Dogs. OMICS, 19(8), 461–470.
- Jiang, L., Wang, J., Li, R., Fang, Z.M., Zhu, X.H., Yi, X., ... Jiang, D.S. (2019). Disturbed energy and amino acid metabolism with their diagnostic potential in mitral valve disease revealed by untargeted plasma metabolic profiling. Metabolomics, 15(4), 57.
- Lanfear, D. E., Gibbs, J. J., Li, J., She, R., Petucci, C., Culver, J. A., … Gardell, S. J. (2017). Targeted Metabolomic Profiling of Plasma and Survival in Heart Failure Patients. Journal of the American College of Cardiology,Heart failure, 5(11), 823–832.
- Oyama, M. A., & Chittur, S. V. (2006). Genomic expression patterns of mitral valve tissues from dogs with degenerative mitral valve disease. American Journal of Veterinary Research, 67(8), 1307–1318.
- Brown, D. A., Perry, J. B., Allen, M. E., Sabbah, H. N., Stauffer, B. L., Shaikh, S. R., … Gheorghiade, M. (2017). Expert consensus document: Mitochondrial function as a therapeutic target in heart failure. Nature reviews. Cardiology, 14(4), 238–250.


